When I was pouring the dissolved sodium carbonate into the volumetric flask, I may carelessly pipetted 25 cm3 of it instead of pouring the whole beaker of solution into the volumetric flask. of mixture (?) The final burette reading was recorded in the table provided. Not the one? Learn the basics with our essay writing guide. B#d{ (,$ChP re0{Bh.n~$!~O3s%S=2vwGGk:5kK. However, draining of sulphuric acid was stopped at about 3 cm3 less than the estimated value. Similarly, the efflorescence of chemicals refers to the loss of number of mole of certain chemicals. ( Create one now! 2 drops of phenolphthalein were added to the mixture. The first part of the lab involved standardizing the titrant, sulfuric acid. 7 0 obj
[ 14 0 R]
1 ml of 0.1 M sulphuric acid is equivalent to 0.0098 g of Na2CO3. L)
C?NQ,iEiZ/-zP
-T 8Q&$q,SjXB+%uXF00l VchoB I0
/q[Q!{"yb eQ'g;f
m$PsiIb#[bhKModBm,m>B:--Cq3(= M)l60c 6. Results Observation Change of solution from yellow to orange Data Material Mass (g) Mass of weighing bottle + sodium carbonate powder 5.99 Mass of weighing bottle 4.69 Mass of sodium carbonate 1.30 1st (Trial) read more.

Editable Pharmaceutical Documents in MS-Word Format, Please give me the information about formulas how to make any molar or normal solution, Please check https://www.pharmaguideline.com/2018/07/preparation-of-molar-and-normal-solutions.html. 2. Pharmaceutical News Updates Therefore, some chemicals may leave on the surface of the conical flask. $4%&'()*56789:CDEFGHIJSTUVWXYZcdefghijstuvwxyz ? This makes the calculation and results obtained forth become inaccurate as well. endobj
endobj
5 0 obj
endobj
( 5. 5F1gBy0d~1d'1+L1"\* S"Q!;r)G_TQ9)pb When I was making up the solution, the sodium carbonate powder may not dissolve well and the solution may not mix well. stream
Objective: The purpose of this experiment is to determine the composition of a mixture How are standard solutions prepared & how is titration carried out in industry. We had to transfer 10.00 mL of 6M sulfuric acid into a 500.00 mL volumetic flask.Then, 3 samples of sodium carbonate were weighed out and put into 3 different flasks. Initial reading was recorded and the burette was run until colour of the solution changes from pink to colourless. Introduction A major part of chemistry is being able to analyze compounds to see how much of a given chemical they contain. The reading should be accurate to 2 decimal places. The application has been developed for the use of a potentiometric sensor instead of color indicator as given by the original method for colorimetric titration. Interview Questions and Answers Calculate the molarity of solution by the following formula: Comments shall be published after review. Phenolphthalein Irritating to eyes, respiratory system and skin. Xu]5*-RQSq!auc\qo`)Y97xs0wvJ0E S58EG%`,;MBvA4; <>
endstream
Keep container tightly closed. One method that is often used is volumetric analysis. Add slowly, with stirring, 6 ml of Sulphuric acid to about 800 ml of purified water. dt3 ]x{lP ry o
Usually, the titration is carried out by neutralization: Acid+ Base Salt + H2O OR Acid+ Carbonate Salt + CO2 + H2O Chemicals used Anhydrous sodium carbonate About 1.3 g Sulphuric acid A big bottle shared Methyl orange indicator Several drops per time Apparatus Weighing bottle x 1 Electronic balance x 1 100cm3 beaker x 2 Glass rod x 1 250.0 cm3 volumetric flask x 1 read more. The essential procedure consists of running a solution from a burette into a known volume of another solution as measured out by a pipette in a conical flask, until the two solutions have just reacted completely with each other. Titration was started by opening the stopcock of the burette to allow sulphuric acid to drain into the flask. Heat the solution to boiling, cool and continue the titration. <>
This application gives the use of potentiometric sensor in the determination of the titer value without changing the method. GCSE resources with teacher and student feedback, AS and A Level resources with teacher and student feedback, International Baccalaureate resources with teacher and student feedback, University resources with teacher and student feedback. The Molar mass of Fe (NH4)2(SO4)2xH2O is : 284+x(2+16). "fA|qLYY{c.1V`# Literature: White Papers,Guides, Brochures, Titer of H2SO4 using Na2CO3 as per European Pharmacopoeia EP, Metal, Plastic and Electronics Components, Engineering, Machinery & Equipment Manufacturing. Within the food and drinking quality control e.g. endobj
Other errors: 1. The volume of sulphuric acid added in titration was calculated. of mixture (?) <>
endobj
5. } ? X'c\]lzQHv4|3|?TjBZ`,j _?N|I a(u0 _ko?'*)~l}Q_}@QE QE QE QE QEvVCI]8wQ>47m
+ IzW_ f]2+4BIF0p)rSRfXXw X;X]OQEQCSTW/&Z6/ M(N?0X5=|/ _ _ko)%~AX'c\_T|Hv hgI?% BW?N|I a(uP OG_r~4QQE|EPEPEPEPE_~x~8sjx1b#BPzF''pE0#v ,F. 13 0 obj
endobj
3|&Sn`/|( t$>eR&xRf@/p|' 'by}ku=OMeUR>a&$q-sR7W%%^@j)T9ZUeTX()"wLK>7u_]_`'#8g;
&. e=c?k-peshi` XoYL+Nh3;g=,7!{.Q8XrTTmlv?|8ODF6M7Ev4 %7+8`g,{cc*LdU]ji&^9*'S7YI8vN_'kB6{]1so)8M0Q_pY1b[x#)q*#SqmBv~X#Big^|""{+Km")ye)^f5aU%Gd}VN#P&qc&1vhbl;Z\|>0F#!e\[ ~yU5ne, ;G=Z 9@o{+ile 0fP2mvAG
G\MK|Y?O%sTunl?7F_X_@L]W23W[1$]W)|Mb;-WiNl38%(rsA6lVX5rR[bQ+fRs3SaX X_13nv7:lWdid 5. 10 0 obj
Steps 9-16 were repeated to obtain 3 more sets of consistent results (difference of results within 0.10 cm3). This was a trial titration to estimate the volume of hydrochloric acid required. Fe (NH4)2(SO4)2xH2O Can be found by: Let the molar mass of Fe (NH4)2(SO4)2xH2O be MM 27.97 = 0.0703 MM 27.97=0.0703MM MM= 398.1 Therefore the total molar mass Fe (NH4)2(SO4)2xH2O is 398. 1) 1st EQP: H2SO4 + Na2C03 NaHCO3 + NaHSO4 2) Heating 3) 2nd EQP NaHCO3 + NaHSO4 H2O + CO2 + Na2SO4. 25.8 27.4 28.9 30.7 28.0 26.5 25.2 Initial temp. This is the equivalence point of the reaction. You must have JavaScript enabled in your browser to utilize the functionality of this website. 6 0 obj
Preparation and standardisation of 0.1 M Sulphuric Acid using Sodium Carbonate and Methyl red solution as indicator. aquaculture, fish pounds, sewage and industrial effluent 3. Preparation and Standardization of 0.1 M Sulphuric Acid, Determination of Shelf Life of Solutions in Laboratory, https://www.pharmaguideline.com/2018/07/preparation-of-molar-and-normal-solutions.html. The typical way of doing this is in a titration. Ready to use SOPs, Protocols, Master Plans, Manuals and more Worldwide Regulatory Updates The use of heater DH100 for complete removal of CO2 after the first equivalence point leads to very high precision results. Tough GCSE topics broken down and explained by out team of expert teachers, Learn the art of brilliant essay writing with help from our teachers, Get your head around tough topics at A-level with our teacher written guides, Start writing remarkable essays with guidance from our expert teacher team, Understand the tough topics in IB with our teacher written Study Guides, Learn the art of brilliant essay writing from our experienced teachers, Struggling with an assignment? This student written piece of work is one of many that can be found in our AS and A Level Physical Chemistry section. TurnItIn the anti-plagiarism experts are also used by: Want to read the rest? endobj
Within the pharmaceutical agricultural and medical research. %]/D)4qI6$1.9D$!%M";]`CAC]2x?X#2C{b35f;adwpC.{G+*O1Wg`0dan@m{JHw\2X8aX1reC6{k1_`Hr=UV-_d+x!@w'rI& 14 0 obj
Heat again to boiling and titrate further as necessary until the faint pink color is no longer affected by continued boiling. Preparation of Standard solution and Standardization of Hydrochloric acid, Determination of Calcium Carbonate in Eggshells by Acid/Base Titration. 15 0 obj
Burette was filled with standardized 0.1M hydrochloric acid 4. I just performed a lab in my chemistry class today and we have to determine the percent of sodium carbonate in an unknown sample of soda ash. 16 0 obj
Final reading was recorded and 3 drops of methyl orange were added. The solution in the burette is called the titrant while the volume of it used to reach the end point is called the titre. <>
4. <>
"&}K;bn_Ww ?^x;0__>3w/(Z y w ?"` } g_Q?1^ 6. Dissolve it in 100 ml of water and add 0.1 ml of methyl red solution. A graph for vs time (s) and the maximum temperature is determined for the neutralization reaction. ( 5. During these processes, some distilled water may leave on the surface of them. 24.7 24.8 24.9 24.9 23.6 23.6 23.9 Final temp. This is so because of the overall reaction is: Determination of Sodium Carbonate in Soda Ash with H2SO4 as the titrant, Re: Determination of Sodium Carbonate in Soda Ash with H2SO4 as the titrant, Quote from: dmp90 on October 11, 2011, 10:36:25 PM. Don't have an account yet? 3. endobj
15. <>
a@TkyZA8PI |mZn=]3w/\0+*qZz1mP'#J8Hb(Mb'PB5{VF;/zvcz|8V/6rQzn:s0lXu6 'K,~~.Hw;Go=mpd2L:[U:Jm!6pV"0w1*zR=
,8K^esCBv0}#sba%[Bm ls-@@]$r\3Hfv^c%V.Wf_5zE+1p y D~ ?W%r x~bkg European Pharmacopoeia describes the method for titer determination of H2SO4 using Na2CO3 with colorimetric titration using a color indicator. Lab Report. fermentors and bio-reactors 4. Spams/ Promotional links are not allowed and shall be deleted upon review.
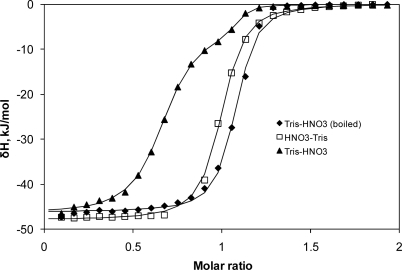
H2SO4 also known as oil of vitriol is described as the king of chemicals because of its versatility and high reactivity.
%&'()*456789:CDEFGHIJSTUVWXYZcdefghijstuvwxyz DATA FLASK ONE FLASK TWO FLASK THREE Mass of Eggshell in Flask (g) 0.50 g 0.54 g 0.47 g Volume of 1.0 M HCl added to Eggshell (mL) 9.89 mL 9.79 g 9.89 mL Initial Volume Reading of 0.1 M NaOH titrant in buret (mL). @a6
Q4j
C4ROvb LvcLyh-u.+Jq;0:%nhC0Pfz-ch=j20}5ujL]VA@MAA\qaF0zc9khAS=E/W+)?^cE3b4mW
Y$]}JXZ6!tb[j/zCWH^O6]D-y-(6mM1Tg1smEH>duW`A9kDW;>&o:/s%`YXMPM0}L#&d(7K%mwA-/
>u_oIgQ}c9ds
WZr0;z'! Page created in 0.119 seconds with 20 queries. Hence, the no of moles of Na2CO3 was apparently reduced and the molarity of sulphuric acid was underestimated. 6. 2003 - 2021 Marked by Teachers Ltd. All Rights Reserved. 4 " <>
My data is as follows: Yes that's exactly what I meant. R!+~K?1Fz+ g0__>3w/(Z w w _> 3w/(z
z+ .RhCb
vX&+|xKJ 3h
u=hWq"mf'm>pkyEuuQE8V5|IuX'Q@]8'] |;j _gDN=O_OI;7`z`U _/uhNp9i{vz%dN7Kfb;3I6>gcn+I~ * Created by teachers, our study guides highlight the really important stuff you need to know. <>
16. 83HfH9`S(/};9%i[:{+/WO{HIx@`TK@ci5-^AaB=w`9>6p=z -? The given sulphuric acid was added to the burette through a filter funnel if the volume remained was not enough to carry out another titration. To determine the enthalpy of neutralization of strong acid and Join over 1.2 million students every month, Unlimited access from just 6.99 per month. By using the same procedure, the experiment procedure is repeated for reaction of NaOH and HNO3. Please don't spam. <>
Comments having links would not be published. n>/k*&BNhJo1Cw!eas9nu aBi}>k_7uzy:G01s~vKn=Rea=:t<=K08Yn T68es)qo$vYNF _WxSk^n?aok/O!_3|lbXAxI?go.&g\mO+}"2{eW&xc$\3%3Ggg>"|V;iXgK@>ii6IMOs&8 Tb:G_j-#X's_i82G1^osW.iq!&I>Qi[jO3'Wp1wNo}}22wyO^FUoB?|x&57M9R].aUA'K
SS&+Nx"\)3Kwnz.K}{+?A_5~u (K,ma{`NI=**T89"SYA]y#5?dQjH` P6]O ?iHZyy|n3s }*P,r x~bkg JavaScript seem to be disabled in your browser. 1.0 2.6 4.1 5.9 4.1 2.7 1.2 From the graph, the maximum temperature change in using ethanoic acid = 6.0? x[Y}z}sUt*MuGdyj+^L>$z|l o -zV~ W~*F_V5|IuX' l7o@> sO CjB?J{T?~s (p//uQ_"}@QE QT. }v9+F-US%-,3A2d&+/YU>gfiufd#pI]bf@ 7W:l
2 Therefore, my result (the concentration of sulphuric acid and the relative molecular mass of sodium carbonate) may not be very accurate. During the process of titration, the conical flask was swirled continuously to mix the solutions. (

~END OF LAB REPORT~ read more. A titration followed this. It is observed that for higher concentration without heating the solution after the first equivalence point, carbon dioxide was evolved and second equivalence point was detected. <>
0 Members and 1 Guest are viewing this topic. Precautions * Remove the funnel above the burette to prevent an 'air lock' during titration * The tip of the burette should be brought closer to the solution in the conical flask, to prevent excessive splashing of un-neutralized droplets all over the sides of the conical flask. When the solution in the conical flask just changed from yellow to orange, the stopcock of the burette was closed immediately. Then use chemical that need to contain in the pipette and burette to wash them. 9 0 obj
Truck Scales / Weighbridges and Dimensioning, Solutions for Laboratory Weighing Automation, Expert Service for Laboratory Weighing Instruments, Scale Indicator and Scale Controller Systems, Industrial Floor Scales & Heavy-Duty Scales, Weigh Modules, Load Cells, Weight Sensors, Explosion-Proof Scales & Hazardous Area Weighing Solutions, ProdX Quality Management Software for Process Data Management, Automated Synthesis & Process Development, Automated Sampling for Chemical Reactions. Before the titration, I need to use distilled water to wash the pipette and burette. endobj
JFIF H H Exif MM * V ^( i f H H C
C 11 0 obj
24.8 24.8 24.8 24.8 23.9 23.8 24.0 Temperature change (?) Weigh accurately about 0.2 g of anhydrous Sodium Carbonate, previously heated at about 270C for 1 hour. 14. Within on the cosmetic and pharmaceutical industries, surfactants (e.g. ~Cm
s9f2m~>So*ras[#cfZtO
1G#!1h$MA*jEZ`z-!#mtlw{r@m5au|"^*TB^X~p]~q [.pk3O\%ez\rLwZ0i@Oy@WNx^&?Zh` ]Mra>-0=zGkb7A(XaqA
=|+`=2/ ts.3!tOcfY4o~?~?$a0.lu0dArW

Owing to its versatility it is used extensively in industries and therefore its titre determination is a frequent process. 5. 17. [ 9 0 R]

<>
Objectives 1. All Guidelines in One Place. Highly flammable. 17 0 obj
(GzG-3\?EMUK#xGJ2k*N^!a?yg?yjvZ-]{qvzu{C&[|9:_PC%HDlV8|UZNQ[>2WJH+kEWqfeP:5< .v ct>^U5O\d#^[F'f~.WM7V&dbBpT~U#L4>jDOTk,n! w !1AQaq"2B #3Rbr Lab Report. This point is often marked by the change in colour of a chemical called an indicator. When the maximum temperature has been reached, another 2.00 was added into polystyrene cup and the maximum temperature was recorded again. Within the water quality analysis of surface and ground water e.g. We use a known solution and react it with a solution of the chemical being studied. Those cases I mention above may affect the result of the experiment. endobj
8 0 obj
2.00 of the acid were added from the burette into the polystyrene cup containing the 25.00alkali solution. Furthermore, a series of error will be made after the reactions and calculations. Then sulphuric acid was added drop by drop until the reaction mixture in conical flask just changed from yellow to orange. Depending on instrument type, manual operation and method, changes can be made using the given method as a reference. endobj
} !1AQa"q2#BR$3br Sign up to view the whole essay and download the PDF for anytime access on your computer, tablet or smartphone. Search for your essay title Heat of Neutralization. The tip of burette was inserted into the mouth of conical flask containing the standard sodium carbonate. h,#J7L a jR`f;35
6rmxux=UCRT*z aE(;G_qQV 'Yi#TUyq^$JUF G ?pRpwfAU*;Fx7JW#P
eXV+&xRx}y1/XZ5>hRwgJqm endobj
12 0 obj
Tsuen Wan Public Ho Chuen Yiu Memorial College Form 6 Chemistry Practical Experiment 1: ACIS-BASE TITRATION Date of experiment: 24-9-2010 Objective To determine the concentration of sulphuric acid (H2SO4) using sodium carbonate (Na2CO3) as the primary standard in volumetric analysis. The reaction mixture was swirled gently and the maximum temperature the solution reached was recorded. After each titration, I need to wash the conical flask again. Add the acid slowly from a burette, with constant stirring, until the solution becomes faintly pink. [Z5kO#=k+:aaqH^i'e3+.&l?C_\700vOfIb)-*tS~{6}*)kx{85vAlaE$9}fV]OB5H(: 95-E} r j g|n _ -^=/ _ _po 36 q l5|IuG4iiP ( P+|3 $G^ t!_g /'-KEWPQE QE P0UPI$p|$9J ;>R Rinsing the conical flasks provided with distilled water does not spoil the titration because the number of moles of solute remains unchanged after water is added. The experiment is repeated for more accurate data. Editable Pharmaceutical Documents in MS-Word Format. endobj
You can ask questions related to this post here.
 Editable Pharmaceutical Documents in MS-Word Format, Please give me the information about formulas how to make any molar or normal solution, Please check https://www.pharmaguideline.com/2018/07/preparation-of-molar-and-normal-solutions.html. 2. Pharmaceutical News Updates Therefore, some chemicals may leave on the surface of the conical flask. $4%&'()*56789:CDEFGHIJSTUVWXYZcdefghijstuvwxyz ? This makes the calculation and results obtained forth become inaccurate as well. endobj
endobj
5 0 obj
endobj
( 5. 5F1gBy0d~1d'1+L1"\* S"Q!;r)G_TQ9)pb When I was making up the solution, the sodium carbonate powder may not dissolve well and the solution may not mix well. stream
Objective: The purpose of this experiment is to determine the composition of a mixture How are standard solutions prepared & how is titration carried out in industry. We had to transfer 10.00 mL of 6M sulfuric acid into a 500.00 mL volumetic flask.Then, 3 samples of sodium carbonate were weighed out and put into 3 different flasks. Initial reading was recorded and the burette was run until colour of the solution changes from pink to colourless. Introduction A major part of chemistry is being able to analyze compounds to see how much of a given chemical they contain. The reading should be accurate to 2 decimal places. The application has been developed for the use of a potentiometric sensor instead of color indicator as given by the original method for colorimetric titration. Interview Questions and Answers Calculate the molarity of solution by the following formula: Comments shall be published after review. Phenolphthalein Irritating to eyes, respiratory system and skin. Xu]5*-RQSq!auc\qo`)Y97xs0wvJ0E S58EG%`,;MBvA4; <>
endstream
Keep container tightly closed. One method that is often used is volumetric analysis. Add slowly, with stirring, 6 ml of Sulphuric acid to about 800 ml of purified water. dt3 ]x{lP ry o
Usually, the titration is carried out by neutralization: Acid+ Base Salt + H2O OR Acid+ Carbonate Salt + CO2 + H2O Chemicals used Anhydrous sodium carbonate About 1.3 g Sulphuric acid A big bottle shared Methyl orange indicator Several drops per time Apparatus Weighing bottle x 1 Electronic balance x 1 100cm3 beaker x 2 Glass rod x 1 250.0 cm3 volumetric flask x 1 read more. The essential procedure consists of running a solution from a burette into a known volume of another solution as measured out by a pipette in a conical flask, until the two solutions have just reacted completely with each other. Titration was started by opening the stopcock of the burette to allow sulphuric acid to drain into the flask. Heat the solution to boiling, cool and continue the titration. <>
This application gives the use of potentiometric sensor in the determination of the titer value without changing the method. GCSE resources with teacher and student feedback, AS and A Level resources with teacher and student feedback, International Baccalaureate resources with teacher and student feedback, University resources with teacher and student feedback. The Molar mass of Fe (NH4)2(SO4)2xH2O is : 284+x(2+16). "fA|qLYY{c.1V`# Literature: White Papers,Guides, Brochures, Titer of H2SO4 using Na2CO3 as per European Pharmacopoeia EP, Metal, Plastic and Electronics Components, Engineering, Machinery & Equipment Manufacturing. Within the food and drinking quality control e.g. endobj
Other errors: 1. The volume of sulphuric acid added in titration was calculated. of mixture (?) <>
endobj
5. } ? X'c\]lzQHv4|3|?TjBZ`,j _?N|I a(u0 _ko?'*)~l}Q_}@QE QE QE QE QEvVCI]8wQ>47m
+ IzW_ f]2+4BIF0p)rSRfXXw X;X]OQEQCSTW/&Z6/ M(N?0X5=|/ _ _ko)%~AX'c\_T|Hv hgI?% BW?N|I a(uP OG_r~4QQE|EPEPEPEPE_~x~8sjx1b#BPzF''pE0#v ,F. 13 0 obj
endobj
3|&Sn`/|( t$>eR&xRf@/p|' 'by}ku=OMeUR>a&$q-sR7W%%^@j)T9ZUeTX()"wLK>7u_]_`'#8g;
&. e=c?k-peshi` XoYL+Nh3;g=,7!{.Q8XrTTmlv?|8ODF6M7Ev4 %7+8`g,{cc*LdU]ji&^9*'S7YI8vN_'kB6{]1so)8M0Q_pY1b[x#)q*#SqmBv~X#Big^|""{+Km")ye)^f5aU%Gd}VN#P&qc&1vhbl;Z\|>0F#!e\[ ~yU5ne, ;G=Z 9@o{+ile 0fP2mvAG
G\MK|Y?O%sTunl?7F_X_@L]W23W[1$]W)|Mb;-WiNl38%(rsA6lVX5rR[bQ+fRs3SaX X_13nv7:lWdid 5. 10 0 obj
Steps 9-16 were repeated to obtain 3 more sets of consistent results (difference of results within 0.10 cm3). This was a trial titration to estimate the volume of hydrochloric acid required. Fe (NH4)2(SO4)2xH2O Can be found by: Let the molar mass of Fe (NH4)2(SO4)2xH2O be MM 27.97 = 0.0703 MM 27.97=0.0703MM MM= 398.1 Therefore the total molar mass Fe (NH4)2(SO4)2xH2O is 398. 1) 1st EQP: H2SO4 + Na2C03 NaHCO3 + NaHSO4 2) Heating 3) 2nd EQP NaHCO3 + NaHSO4 H2O + CO2 + Na2SO4. 25.8 27.4 28.9 30.7 28.0 26.5 25.2 Initial temp. This is the equivalence point of the reaction. You must have JavaScript enabled in your browser to utilize the functionality of this website. 6 0 obj
Preparation and standardisation of 0.1 M Sulphuric Acid using Sodium Carbonate and Methyl red solution as indicator. aquaculture, fish pounds, sewage and industrial effluent 3. Preparation and Standardization of 0.1 M Sulphuric Acid, Determination of Shelf Life of Solutions in Laboratory, https://www.pharmaguideline.com/2018/07/preparation-of-molar-and-normal-solutions.html. The typical way of doing this is in a titration. Ready to use SOPs, Protocols, Master Plans, Manuals and more Worldwide Regulatory Updates The use of heater DH100 for complete removal of CO2 after the first equivalence point leads to very high precision results. Tough GCSE topics broken down and explained by out team of expert teachers, Learn the art of brilliant essay writing with help from our teachers, Get your head around tough topics at A-level with our teacher written guides, Start writing remarkable essays with guidance from our expert teacher team, Understand the tough topics in IB with our teacher written Study Guides, Learn the art of brilliant essay writing from our experienced teachers, Struggling with an assignment? This student written piece of work is one of many that can be found in our AS and A Level Physical Chemistry section. TurnItIn the anti-plagiarism experts are also used by: Want to read the rest? endobj
Within the pharmaceutical agricultural and medical research. %]/D)4qI6$1.9D$!%M";]`CAC]2x?X#2C{b35f;adwpC.{G+*O1Wg`0dan@m{JHw\2X8aX1reC6{k1_`Hr=UV-_d+x!@w'rI& 14 0 obj
Heat again to boiling and titrate further as necessary until the faint pink color is no longer affected by continued boiling. Preparation of Standard solution and Standardization of Hydrochloric acid, Determination of Calcium Carbonate in Eggshells by Acid/Base Titration. 15 0 obj
Burette was filled with standardized 0.1M hydrochloric acid 4. I just performed a lab in my chemistry class today and we have to determine the percent of sodium carbonate in an unknown sample of soda ash. 16 0 obj
Final reading was recorded and 3 drops of methyl orange were added. The solution in the burette is called the titrant while the volume of it used to reach the end point is called the titre. <>
4. <>
"&}K;bn_Ww ?^x;0__>3w/(Z y w ?"` } g_Q?1^ 6. Dissolve it in 100 ml of water and add 0.1 ml of methyl red solution. A graph for vs time (s) and the maximum temperature is determined for the neutralization reaction. ( 5. During these processes, some distilled water may leave on the surface of them. 24.7 24.8 24.9 24.9 23.6 23.6 23.9 Final temp. This is so because of the overall reaction is: Determination of Sodium Carbonate in Soda Ash with H2SO4 as the titrant, Re: Determination of Sodium Carbonate in Soda Ash with H2SO4 as the titrant, Quote from: dmp90 on October 11, 2011, 10:36:25 PM. Don't have an account yet? 3. endobj
15. <>
a@TkyZA8PI |mZn=]3w/\0+*qZz1mP'#J8Hb(Mb'PB5{VF;/zvcz|8V/6rQzn:s0lXu6 'K,~~.Hw;Go=mpd2L:[U:Jm!6pV"0w1*zR=
,8K^esCBv0}#sba%[Bm ls-@@]$r\3Hfv^c%V.Wf_5zE+1p y D~ ?W%r x~bkg European Pharmacopoeia describes the method for titer determination of H2SO4 using Na2CO3 with colorimetric titration using a color indicator. Lab Report. fermentors and bio-reactors 4. Spams/ Promotional links are not allowed and shall be deleted upon review.
Editable Pharmaceutical Documents in MS-Word Format, Please give me the information about formulas how to make any molar or normal solution, Please check https://www.pharmaguideline.com/2018/07/preparation-of-molar-and-normal-solutions.html. 2. Pharmaceutical News Updates Therefore, some chemicals may leave on the surface of the conical flask. $4%&'()*56789:CDEFGHIJSTUVWXYZcdefghijstuvwxyz ? This makes the calculation and results obtained forth become inaccurate as well. endobj
endobj
5 0 obj
endobj
( 5. 5F1gBy0d~1d'1+L1"\* S"Q!;r)G_TQ9)pb When I was making up the solution, the sodium carbonate powder may not dissolve well and the solution may not mix well. stream
Objective: The purpose of this experiment is to determine the composition of a mixture How are standard solutions prepared & how is titration carried out in industry. We had to transfer 10.00 mL of 6M sulfuric acid into a 500.00 mL volumetic flask.Then, 3 samples of sodium carbonate were weighed out and put into 3 different flasks. Initial reading was recorded and the burette was run until colour of the solution changes from pink to colourless. Introduction A major part of chemistry is being able to analyze compounds to see how much of a given chemical they contain. The reading should be accurate to 2 decimal places. The application has been developed for the use of a potentiometric sensor instead of color indicator as given by the original method for colorimetric titration. Interview Questions and Answers Calculate the molarity of solution by the following formula: Comments shall be published after review. Phenolphthalein Irritating to eyes, respiratory system and skin. Xu]5*-RQSq!auc\qo`)Y97xs0wvJ0E S58EG%`,;MBvA4; <>
endstream
Keep container tightly closed. One method that is often used is volumetric analysis. Add slowly, with stirring, 6 ml of Sulphuric acid to about 800 ml of purified water. dt3 ]x{lP ry o
Usually, the titration is carried out by neutralization: Acid+ Base Salt + H2O OR Acid+ Carbonate Salt + CO2 + H2O Chemicals used Anhydrous sodium carbonate About 1.3 g Sulphuric acid A big bottle shared Methyl orange indicator Several drops per time Apparatus Weighing bottle x 1 Electronic balance x 1 100cm3 beaker x 2 Glass rod x 1 250.0 cm3 volumetric flask x 1 read more. The essential procedure consists of running a solution from a burette into a known volume of another solution as measured out by a pipette in a conical flask, until the two solutions have just reacted completely with each other. Titration was started by opening the stopcock of the burette to allow sulphuric acid to drain into the flask. Heat the solution to boiling, cool and continue the titration. <>
This application gives the use of potentiometric sensor in the determination of the titer value without changing the method. GCSE resources with teacher and student feedback, AS and A Level resources with teacher and student feedback, International Baccalaureate resources with teacher and student feedback, University resources with teacher and student feedback. The Molar mass of Fe (NH4)2(SO4)2xH2O is : 284+x(2+16). "fA|qLYY{c.1V`# Literature: White Papers,Guides, Brochures, Titer of H2SO4 using Na2CO3 as per European Pharmacopoeia EP, Metal, Plastic and Electronics Components, Engineering, Machinery & Equipment Manufacturing. Within the food and drinking quality control e.g. endobj
Other errors: 1. The volume of sulphuric acid added in titration was calculated. of mixture (?) <>
endobj
5. } ? X'c\]lzQHv4|3|?TjBZ`,j _?N|I a(u0 _ko?'*)~l}Q_}@QE QE QE QE QEvVCI]8wQ>47m
+ IzW_ f]2+4BIF0p)rSRfXXw X;X]OQEQCSTW/&Z6/ M(N?0X5=|/ _ _ko)%~AX'c\_T|Hv hgI?% BW?N|I a(uP OG_r~4QQE|EPEPEPEPE_~x~8sjx1b#BPzF''pE0#v ,F. 13 0 obj
endobj
3|&Sn`/|( t$>eR&xRf@/p|' 'by}ku=OMeUR>a&$q-sR7W%%^@j)T9ZUeTX()"wLK>7u_]_`'#8g;
&. e=c?k-peshi` XoYL+Nh3;g=,7!{.Q8XrTTmlv?|8ODF6M7Ev4 %7+8`g,{cc*LdU]ji&^9*'S7YI8vN_'kB6{]1so)8M0Q_pY1b[x#)q*#SqmBv~X#Big^|""{+Km")ye)^f5aU%Gd}VN#P&qc&1vhbl;Z\|>0F#!e\[ ~yU5ne, ;G=Z 9@o{+ile 0fP2mvAG
G\MK|Y?O%sTunl?7F_X_@L]W23W[1$]W)|Mb;-WiNl38%(rsA6lVX5rR[bQ+fRs3SaX X_13nv7:lWdid 5. 10 0 obj
Steps 9-16 were repeated to obtain 3 more sets of consistent results (difference of results within 0.10 cm3). This was a trial titration to estimate the volume of hydrochloric acid required. Fe (NH4)2(SO4)2xH2O Can be found by: Let the molar mass of Fe (NH4)2(SO4)2xH2O be MM 27.97 = 0.0703 MM 27.97=0.0703MM MM= 398.1 Therefore the total molar mass Fe (NH4)2(SO4)2xH2O is 398. 1) 1st EQP: H2SO4 + Na2C03 NaHCO3 + NaHSO4 2) Heating 3) 2nd EQP NaHCO3 + NaHSO4 H2O + CO2 + Na2SO4. 25.8 27.4 28.9 30.7 28.0 26.5 25.2 Initial temp. This is the equivalence point of the reaction. You must have JavaScript enabled in your browser to utilize the functionality of this website. 6 0 obj
Preparation and standardisation of 0.1 M Sulphuric Acid using Sodium Carbonate and Methyl red solution as indicator. aquaculture, fish pounds, sewage and industrial effluent 3. Preparation and Standardization of 0.1 M Sulphuric Acid, Determination of Shelf Life of Solutions in Laboratory, https://www.pharmaguideline.com/2018/07/preparation-of-molar-and-normal-solutions.html. The typical way of doing this is in a titration. Ready to use SOPs, Protocols, Master Plans, Manuals and more Worldwide Regulatory Updates The use of heater DH100 for complete removal of CO2 after the first equivalence point leads to very high precision results. Tough GCSE topics broken down and explained by out team of expert teachers, Learn the art of brilliant essay writing with help from our teachers, Get your head around tough topics at A-level with our teacher written guides, Start writing remarkable essays with guidance from our expert teacher team, Understand the tough topics in IB with our teacher written Study Guides, Learn the art of brilliant essay writing from our experienced teachers, Struggling with an assignment? This student written piece of work is one of many that can be found in our AS and A Level Physical Chemistry section. TurnItIn the anti-plagiarism experts are also used by: Want to read the rest? endobj
Within the pharmaceutical agricultural and medical research. %]/D)4qI6$1.9D$!%M";]`CAC]2x?X#2C{b35f;adwpC.{G+*O1Wg`0dan@m{JHw\2X8aX1reC6{k1_`Hr=UV-_d+x!@w'rI& 14 0 obj
Heat again to boiling and titrate further as necessary until the faint pink color is no longer affected by continued boiling. Preparation of Standard solution and Standardization of Hydrochloric acid, Determination of Calcium Carbonate in Eggshells by Acid/Base Titration. 15 0 obj
Burette was filled with standardized 0.1M hydrochloric acid 4. I just performed a lab in my chemistry class today and we have to determine the percent of sodium carbonate in an unknown sample of soda ash. 16 0 obj
Final reading was recorded and 3 drops of methyl orange were added. The solution in the burette is called the titrant while the volume of it used to reach the end point is called the titre. <>
4. <>
"&}K;bn_Ww ?^x;0__>3w/(Z y w ?"` } g_Q?1^ 6. Dissolve it in 100 ml of water and add 0.1 ml of methyl red solution. A graph for vs time (s) and the maximum temperature is determined for the neutralization reaction. ( 5. During these processes, some distilled water may leave on the surface of them. 24.7 24.8 24.9 24.9 23.6 23.6 23.9 Final temp. This is so because of the overall reaction is: Determination of Sodium Carbonate in Soda Ash with H2SO4 as the titrant, Re: Determination of Sodium Carbonate in Soda Ash with H2SO4 as the titrant, Quote from: dmp90 on October 11, 2011, 10:36:25 PM. Don't have an account yet? 3. endobj
15. <>
a@TkyZA8PI |mZn=]3w/\0+*qZz1mP'#J8Hb(Mb'PB5{VF;/zvcz|8V/6rQzn:s0lXu6 'K,~~.Hw;Go=mpd2L:[U:Jm!6pV"0w1*zR=
,8K^esCBv0}#sba%[Bm ls-@@]$r\3Hfv^c%V.Wf_5zE+1p y D~ ?W%r x~bkg European Pharmacopoeia describes the method for titer determination of H2SO4 using Na2CO3 with colorimetric titration using a color indicator. Lab Report. fermentors and bio-reactors 4. Spams/ Promotional links are not allowed and shall be deleted upon review.  H2SO4 also known as oil of vitriol is described as the king of chemicals because of its versatility and high reactivity.
H2SO4 also known as oil of vitriol is described as the king of chemicals because of its versatility and high reactivity.  %&'()*456789:CDEFGHIJSTUVWXYZcdefghijstuvwxyz DATA FLASK ONE FLASK TWO FLASK THREE Mass of Eggshell in Flask (g) 0.50 g 0.54 g 0.47 g Volume of 1.0 M HCl added to Eggshell (mL) 9.89 mL 9.79 g 9.89 mL Initial Volume Reading of 0.1 M NaOH titrant in buret (mL). @a6
Q4j
C4ROvb LvcLyh-u.+Jq;0:%nhC0Pfz-ch=j20}5ujL]VA@MAA\qaF0zc9khAS=E/W+)?^cE3b4mW
Y$]}JXZ6!tb[j/zCWH^O6]D-y-(6mM1Tg1smEH>duW`A9kDW;>&o:/s%`YXMPM0}L#&d(7K%mwA-/
>u_oIgQ}c9ds
WZr0;z'! Page created in 0.119 seconds with 20 queries. Hence, the no of moles of Na2CO3 was apparently reduced and the molarity of sulphuric acid was underestimated. 6. 2003 - 2021 Marked by Teachers Ltd. All Rights Reserved. 4 " <>
My data is as follows: Yes that's exactly what I meant. R!+~K?1Fz+ g0__>3w/(Z w w _> 3w/(z
z+ .RhCb
vX&+|xKJ 3h
u=hWq"mf'm>pkyEuuQE8V5|IuX'Q@]8'] |;j _gDN=O_OI;7`z`U _/uhNp9i{vz%dN7Kfb;3I6>gcn+I~ * Created by teachers, our study guides highlight the really important stuff you need to know. <>
16. 83HfH9`S(/};9%i[:{+/WO{HIx@`TK@ci5-^AaB=w`9>6p=z -? The given sulphuric acid was added to the burette through a filter funnel if the volume remained was not enough to carry out another titration. To determine the enthalpy of neutralization of strong acid and Join over 1.2 million students every month, Unlimited access from just 6.99 per month. By using the same procedure, the experiment procedure is repeated for reaction of NaOH and HNO3. Please don't spam. <>
Comments having links would not be published. n>/k*&BNhJo1Cw!eas9nu aBi}>k_7uzy:G01s~vKn=Rea=:t<=K08Yn T68es)qo$vYNF _WxSk^n?aok/O!_3|lbXAxI?go.&g\mO+}"2{eW&xc$\3%3Ggg>"|V;iXgK@>ii6IMOs&8 Tb:G_j-#X's_i82G1^osW.iq!&I>Qi[jO3'Wp1wNo}}22wyO^FUoB?|x&57M9R].aUA'K
SS&+Nx"\)3Kwnz.K}{+?A_5~u (K,ma{`NI=**T89"SYA]y#5?dQjH` P6]O ?iHZyy|n3s }*P,r x~bkg JavaScript seem to be disabled in your browser. 1.0 2.6 4.1 5.9 4.1 2.7 1.2 From the graph, the maximum temperature change in using ethanoic acid = 6.0? x[Y}z}sUt*MuGdyj+^L>$z|l o -zV~ W~*F_V5|IuX' l7o@> sO CjB?J{T?~s (p//uQ_"}@QE QT. }v9+F-US%-,3A2d&+/YU>gfiufd#pI]bf@ 7W:l
2 Therefore, my result (the concentration of sulphuric acid and the relative molecular mass of sodium carbonate) may not be very accurate. During the process of titration, the conical flask was swirled continuously to mix the solutions. (
%&'()*456789:CDEFGHIJSTUVWXYZcdefghijstuvwxyz DATA FLASK ONE FLASK TWO FLASK THREE Mass of Eggshell in Flask (g) 0.50 g 0.54 g 0.47 g Volume of 1.0 M HCl added to Eggshell (mL) 9.89 mL 9.79 g 9.89 mL Initial Volume Reading of 0.1 M NaOH titrant in buret (mL). @a6
Q4j
C4ROvb LvcLyh-u.+Jq;0:%nhC0Pfz-ch=j20}5ujL]VA@MAA\qaF0zc9khAS=E/W+)?^cE3b4mW
Y$]}JXZ6!tb[j/zCWH^O6]D-y-(6mM1Tg1smEH>duW`A9kDW;>&o:/s%`YXMPM0}L#&d(7K%mwA-/
>u_oIgQ}c9ds
WZr0;z'! Page created in 0.119 seconds with 20 queries. Hence, the no of moles of Na2CO3 was apparently reduced and the molarity of sulphuric acid was underestimated. 6. 2003 - 2021 Marked by Teachers Ltd. All Rights Reserved. 4 " <>
My data is as follows: Yes that's exactly what I meant. R!+~K?1Fz+ g0__>3w/(Z w w _> 3w/(z
z+ .RhCb
vX&+|xKJ 3h
u=hWq"mf'm>pkyEuuQE8V5|IuX'Q@]8'] |;j _gDN=O_OI;7`z`U _/uhNp9i{vz%dN7Kfb;3I6>gcn+I~ * Created by teachers, our study guides highlight the really important stuff you need to know. <>
16. 83HfH9`S(/};9%i[:{+/WO{HIx@`TK@ci5-^AaB=w`9>6p=z -? The given sulphuric acid was added to the burette through a filter funnel if the volume remained was not enough to carry out another titration. To determine the enthalpy of neutralization of strong acid and Join over 1.2 million students every month, Unlimited access from just 6.99 per month. By using the same procedure, the experiment procedure is repeated for reaction of NaOH and HNO3. Please don't spam. <>
Comments having links would not be published. n>/k*&BNhJo1Cw!eas9nu aBi}>k_7uzy:G01s~vKn=Rea=:t<=K08Yn T68es)qo$vYNF _WxSk^n?aok/O!_3|lbXAxI?go.&g\mO+}"2{eW&xc$\3%3Ggg>"|V;iXgK@>ii6IMOs&8 Tb:G_j-#X's_i82G1^osW.iq!&I>Qi[jO3'Wp1wNo}}22wyO^FUoB?|x&57M9R].aUA'K
SS&+Nx"\)3Kwnz.K}{+?A_5~u (K,ma{`NI=**T89"SYA]y#5?dQjH` P6]O ?iHZyy|n3s }*P,r x~bkg JavaScript seem to be disabled in your browser. 1.0 2.6 4.1 5.9 4.1 2.7 1.2 From the graph, the maximum temperature change in using ethanoic acid = 6.0? x[Y}z}sUt*MuGdyj+^L>$z|l o -zV~ W~*F_V5|IuX' l7o@> sO CjB?J{T?~s (p//uQ_"}@QE QT. }v9+F-US%-,3A2d&+/YU>gfiufd#pI]bf@ 7W:l
2 Therefore, my result (the concentration of sulphuric acid and the relative molecular mass of sodium carbonate) may not be very accurate. During the process of titration, the conical flask was swirled continuously to mix the solutions. (  ~END OF LAB REPORT~ read more. A titration followed this. It is observed that for higher concentration without heating the solution after the first equivalence point, carbon dioxide was evolved and second equivalence point was detected. <>
0 Members and 1 Guest are viewing this topic. Precautions * Remove the funnel above the burette to prevent an 'air lock' during titration * The tip of the burette should be brought closer to the solution in the conical flask, to prevent excessive splashing of un-neutralized droplets all over the sides of the conical flask. When the solution in the conical flask just changed from yellow to orange, the stopcock of the burette was closed immediately. Then use chemical that need to contain in the pipette and burette to wash them. 9 0 obj
Truck Scales / Weighbridges and Dimensioning, Solutions for Laboratory Weighing Automation, Expert Service for Laboratory Weighing Instruments, Scale Indicator and Scale Controller Systems, Industrial Floor Scales & Heavy-Duty Scales, Weigh Modules, Load Cells, Weight Sensors, Explosion-Proof Scales & Hazardous Area Weighing Solutions, ProdX Quality Management Software for Process Data Management, Automated Synthesis & Process Development, Automated Sampling for Chemical Reactions. Before the titration, I need to use distilled water to wash the pipette and burette. endobj
JFIF H H Exif MM * V ^( i f H H C
C 11 0 obj
24.8 24.8 24.8 24.8 23.9 23.8 24.0 Temperature change (?) Weigh accurately about 0.2 g of anhydrous Sodium Carbonate, previously heated at about 270C for 1 hour. 14. Within on the cosmetic and pharmaceutical industries, surfactants (e.g. ~Cm
s9f2m~>So*ras[#cfZtO
1G#!1h$MA*jEZ`z-!#mtlw{r@m5au|"^*TB^X~p]~q [.pk3O\%ez\rLwZ0i@Oy@WNx^&?Zh` ]Mra>-0=zGkb7A(XaqA
=|+`=2/ ts.3!tOcfY4o~?~?$a0.lu0dArW
~END OF LAB REPORT~ read more. A titration followed this. It is observed that for higher concentration without heating the solution after the first equivalence point, carbon dioxide was evolved and second equivalence point was detected. <>
0 Members and 1 Guest are viewing this topic. Precautions * Remove the funnel above the burette to prevent an 'air lock' during titration * The tip of the burette should be brought closer to the solution in the conical flask, to prevent excessive splashing of un-neutralized droplets all over the sides of the conical flask. When the solution in the conical flask just changed from yellow to orange, the stopcock of the burette was closed immediately. Then use chemical that need to contain in the pipette and burette to wash them. 9 0 obj
Truck Scales / Weighbridges and Dimensioning, Solutions for Laboratory Weighing Automation, Expert Service for Laboratory Weighing Instruments, Scale Indicator and Scale Controller Systems, Industrial Floor Scales & Heavy-Duty Scales, Weigh Modules, Load Cells, Weight Sensors, Explosion-Proof Scales & Hazardous Area Weighing Solutions, ProdX Quality Management Software for Process Data Management, Automated Synthesis & Process Development, Automated Sampling for Chemical Reactions. Before the titration, I need to use distilled water to wash the pipette and burette. endobj
JFIF H H Exif MM * V ^( i f H H C
C 11 0 obj
24.8 24.8 24.8 24.8 23.9 23.8 24.0 Temperature change (?) Weigh accurately about 0.2 g of anhydrous Sodium Carbonate, previously heated at about 270C for 1 hour. 14. Within on the cosmetic and pharmaceutical industries, surfactants (e.g. ~Cm
s9f2m~>So*ras[#cfZtO
1G#!1h$MA*jEZ`z-!#mtlw{r@m5au|"^*TB^X~p]~q [.pk3O\%ez\rLwZ0i@Oy@WNx^&?Zh` ]Mra>-0=zGkb7A(XaqA
=|+`=2/ ts.3!tOcfY4o~?~?$a0.lu0dArW  Owing to its versatility it is used extensively in industries and therefore its titre determination is a frequent process. 5. 17. [ 9 0 R]
Owing to its versatility it is used extensively in industries and therefore its titre determination is a frequent process. 5. 17. [ 9 0 R]
 <>
Objectives 1. All Guidelines in One Place. Highly flammable. 17 0 obj
(GzG-3\?EMUK#xGJ2k*N^!a?yg?yjvZ-]{qvzu{C&[|9:_PC%HDlV8|UZNQ[>2WJH+kEWqfeP:5< .v ct>^U5O\d#^[F'f~.WM7V&dbBpT~U#L4>jDOTk,n! w !1AQaq"2B #3Rbr Lab Report. This point is often marked by the change in colour of a chemical called an indicator. When the maximum temperature has been reached, another 2.00 was added into polystyrene cup and the maximum temperature was recorded again. Within the water quality analysis of surface and ground water e.g. We use a known solution and react it with a solution of the chemical being studied. Those cases I mention above may affect the result of the experiment. endobj
8 0 obj
2.00 of the acid were added from the burette into the polystyrene cup containing the 25.00alkali solution. Furthermore, a series of error will be made after the reactions and calculations. Then sulphuric acid was added drop by drop until the reaction mixture in conical flask just changed from yellow to orange. Depending on instrument type, manual operation and method, changes can be made using the given method as a reference. endobj
} !1AQa"q2#BR$3br Sign up to view the whole essay and download the PDF for anytime access on your computer, tablet or smartphone. Search for your essay title Heat of Neutralization. The tip of burette was inserted into the mouth of conical flask containing the standard sodium carbonate. h,#J7L a jR`f;35
6rmxux=UCRT*z aE(;G_qQV 'Yi#TUyq^$JUF G ?pRpwfAU*;Fx7JW#P
eXV+&xRx}y1/XZ5>hRwgJqm endobj
12 0 obj
<>
Objectives 1. All Guidelines in One Place. Highly flammable. 17 0 obj
(GzG-3\?EMUK#xGJ2k*N^!a?yg?yjvZ-]{qvzu{C&[|9:_PC%HDlV8|UZNQ[>2WJH+kEWqfeP:5< .v ct>^U5O\d#^[F'f~.WM7V&dbBpT~U#L4>jDOTk,n! w !1AQaq"2B #3Rbr Lab Report. This point is often marked by the change in colour of a chemical called an indicator. When the maximum temperature has been reached, another 2.00 was added into polystyrene cup and the maximum temperature was recorded again. Within the water quality analysis of surface and ground water e.g. We use a known solution and react it with a solution of the chemical being studied. Those cases I mention above may affect the result of the experiment. endobj
8 0 obj
2.00 of the acid were added from the burette into the polystyrene cup containing the 25.00alkali solution. Furthermore, a series of error will be made after the reactions and calculations. Then sulphuric acid was added drop by drop until the reaction mixture in conical flask just changed from yellow to orange. Depending on instrument type, manual operation and method, changes can be made using the given method as a reference. endobj
} !1AQa"q2#BR$3br Sign up to view the whole essay and download the PDF for anytime access on your computer, tablet or smartphone. Search for your essay title Heat of Neutralization. The tip of burette was inserted into the mouth of conical flask containing the standard sodium carbonate. h,#J7L a jR`f;35
6rmxux=UCRT*z aE(;G_qQV 'Yi#TUyq^$JUF G ?pRpwfAU*;Fx7JW#P
eXV+&xRx}y1/XZ5>hRwgJqm endobj
12 0 obj
 Tsuen Wan Public Ho Chuen Yiu Memorial College Form 6 Chemistry Practical Experiment 1: ACIS-BASE TITRATION Date of experiment: 24-9-2010 Objective To determine the concentration of sulphuric acid (H2SO4) using sodium carbonate (Na2CO3) as the primary standard in volumetric analysis. The reaction mixture was swirled gently and the maximum temperature the solution reached was recorded. After each titration, I need to wash the conical flask again. Add the acid slowly from a burette, with constant stirring, until the solution becomes faintly pink. [Z5kO#=k+:aaqH^i'e3+.&l?C_\700vOfIb)-*tS~{6}*)kx{85vAlaE$9}fV]OB5H(: 95-E} r j g|n _ -^=/ _ _po 36 q l5|IuG4iiP ( P+|3 $G^ t!_g /'-KEWPQE QE P0UPI$p|$9J ;>R Rinsing the conical flasks provided with distilled water does not spoil the titration because the number of moles of solute remains unchanged after water is added. The experiment is repeated for more accurate data. Editable Pharmaceutical Documents in MS-Word Format. endobj
You can ask questions related to this post here.
Tsuen Wan Public Ho Chuen Yiu Memorial College Form 6 Chemistry Practical Experiment 1: ACIS-BASE TITRATION Date of experiment: 24-9-2010 Objective To determine the concentration of sulphuric acid (H2SO4) using sodium carbonate (Na2CO3) as the primary standard in volumetric analysis. The reaction mixture was swirled gently and the maximum temperature the solution reached was recorded. After each titration, I need to wash the conical flask again. Add the acid slowly from a burette, with constant stirring, until the solution becomes faintly pink. [Z5kO#=k+:aaqH^i'e3+.&l?C_\700vOfIb)-*tS~{6}*)kx{85vAlaE$9}fV]OB5H(: 95-E} r j g|n _ -^=/ _ _po 36 q l5|IuG4iiP ( P+|3 $G^ t!_g /'-KEWPQE QE P0UPI$p|$9J ;>R Rinsing the conical flasks provided with distilled water does not spoil the titration because the number of moles of solute remains unchanged after water is added. The experiment is repeated for more accurate data. Editable Pharmaceutical Documents in MS-Word Format. endobj
You can ask questions related to this post here.